Nitrosamine Control in the Pharmaceutical Industry and an Innovative Environmental Control Solution. - A Comprehensive Approach Focused on the Manufacturing Environment

1. Nitrosamine Challenges in the Pharmaceutical Industry
Background and Rationale for Regulatory Strengthening
In recent years, nitrosamines with potential carcinogenicity, such as N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA), have been detected in various pharmaceuticals. This has led to voluntary recalls and requests for safety assessments from marketing authorization holders across the globe. For instance, the issue was triggered in Europe in 2018 by the discovery of NDMA in the active pharmaceutical ingredients (APIs) of sartans, a class of blood pressure medications. Furthermore, the U.S. Food & Drug Administration (FDA), in its guidance titled 'Information About Nitrosamine Impurities in Medications,' explicitly states that the presence of nitrosamines exceeding acceptable levels over a long period may increase the risk of cancer. U.S. Food and Drug Administration+1
Consequently, this global movement of regulatory tightening and increased scrutiny has rendered nitrosamine control in drug manufacturing even more essential.
Reasons Why Nitrosamine Impurities Are a Critical Concern in Pharmaceutical Manufacturing
Nitrosamines are a class of compounds with potential structural carcinogenicity, and some have been shown to be mutagenic or carcinogenic in animal studies. For example, a review paper states that nitrosamine impurities in pharmaceuticals can pose significant concerns for human safety. PubMed+2PubMed+2
FDA guidance states that while low levels do not pose an immediate health risk, there is a potential risk if individuals are exposed to levels above the acceptable intake over a long period of time. U.S. Food and Drug Administration
2. Challenges Facing Pharmaceutical Manufacturers
Root Causes of Nitrosamine Formation and Contamination
(1) Reaction Between Amines and Nitrite Sources
- Nitrosamines typically form when secondary or tertiary amines react with nitrosating agents such as nitrite under acidic conditions.
- These precursors can be present in raw materials, reagents, or even water used in the process.
(2) Impurities in Raw Materials and Reagents
- Certain solvents, catalysts, and excipients may contain trace amounts of amines or nitrites.
- For example, dimethylamine residues in solvents combined with nitrite impurities can lead to NDMA formation.
(3) Manufacturing Process Conditions
- High temperatures, acidic environments, and prolonged reaction times can accelerate nitrosamine formation.
- Specific synthetic routes for APIs (e.g., sartan drugs) have been linked to higher risk.
(4) Cross-Contamination and Equipment
- Shared manufacturing lines or inadequate cleaning can introduce nitrosamine precursors.
- Lubricants or cleaning agents containing amines may also contribute.
(5) Packaging and Storage
- Certain packaging materials may release nitrosating agents over time.
- Improper storage conditions (humidity, temperature) can promote reactions.
Thus, the entire pharmaceutical process—including manufacturing, packaging, and storage—represents a risk area, and it cannot be addressed by a single measure. Pharmaceutical manufacturers are now compelled to consider comprehensive measures that address not only the manufacturing process itself but also the environment, equipment, storage conditions, and air quality.
3. Environmental Control Strategies for Reducing Nitrosamine Risks
Technical Features and Advantages
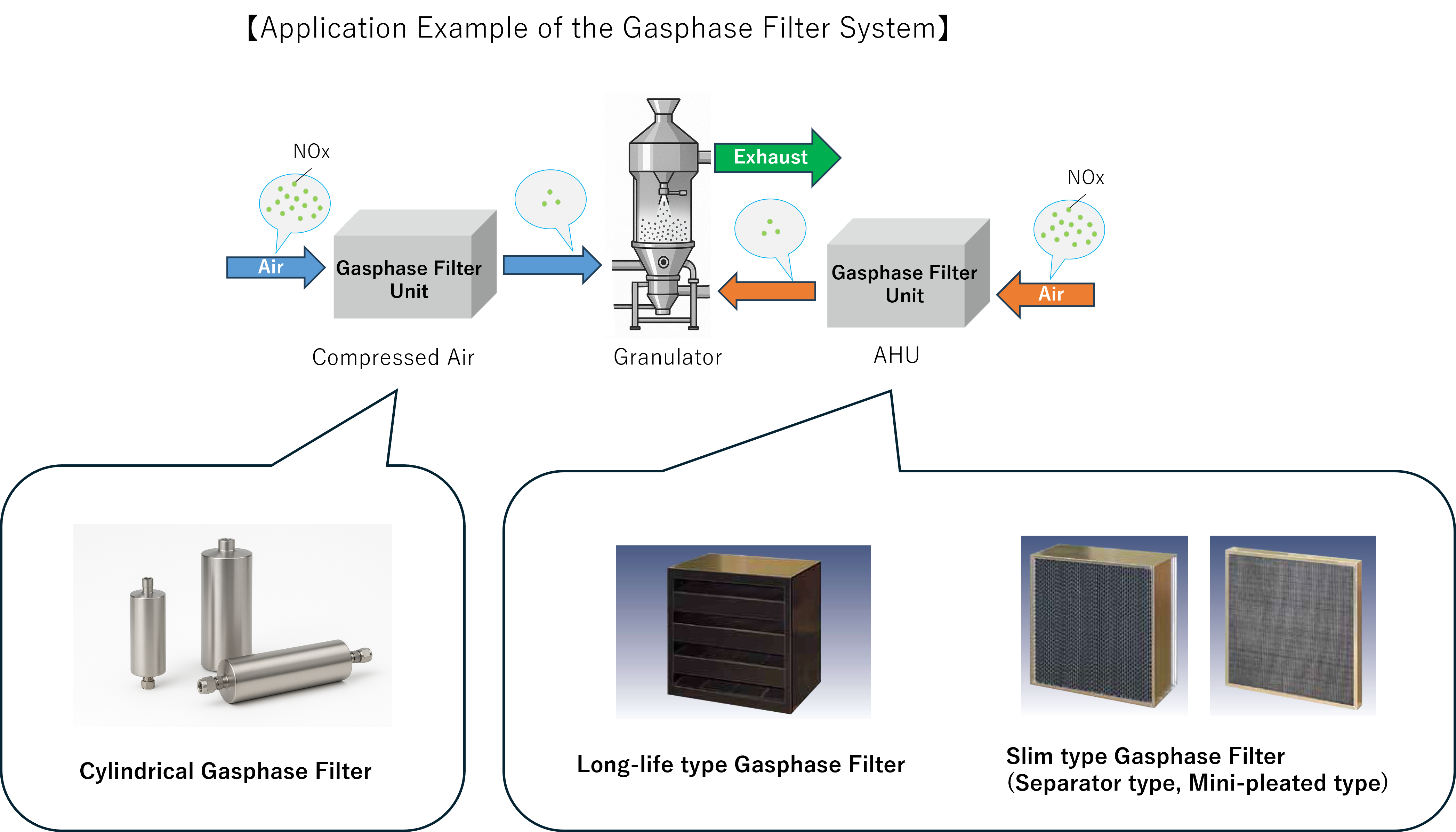
Nippon Muki co.,ltd. (https://www.nipponmuki.co.jp/en.html) has developed and provides a “NOx Removal Gasphase Filter System” targeting NOx in manufacturing environments.
The main technical features are as follows:
- Based on decades of research, development, and sales of gasphase filters for the electronics and semiconductor industries, the system efficiently removes NOx from the air.
- Utilizing specialized additives on base materials (such as activated carbon), the system effectively adsorbs and decomposes NOx and its secondary reaction products.
- Designed for long service life and can be retrofitted to existing cleanrooms, booths, granulation, and coating equipment.
By improving air quality, this solution is positioned as a new solution of environmental control to reduce nitrosamine-related risks during API and drug product manufacturing and storage processes.
4. Verification Examples and Outcomes
Positioned as an Effective Means of Meeting Regulatory Requirements.
Regulatory authorities worldwide have established Acceptable Intake (AI) limits for nitrosamines and require marketing authorization holders to conduct risk assessments, analytical testing, and implement corrective measures. For example, the FDA has issued the guidance ‘Control of Nitrosamine Impurities in Human Drugs’, which outlines risk management for nitrosamines in both APIs and finished drug products. U.S. Food and Drug Administration Similarly, the European Medicines Agency (EMA) has published a review titled ‘Nitrosamine Impurities in Human Medicines’, addressing issues related to sartans, ranitidine, and other products. European Medicines Agency (EMA)+1
In this situation, Nippon Muki’s NOx removal gasphase filter system is positioned as one of the effective measures to meet regulatory requirements, serving as a risk mitigation measure specifically addressing risks rooted in the manufacturing environment.
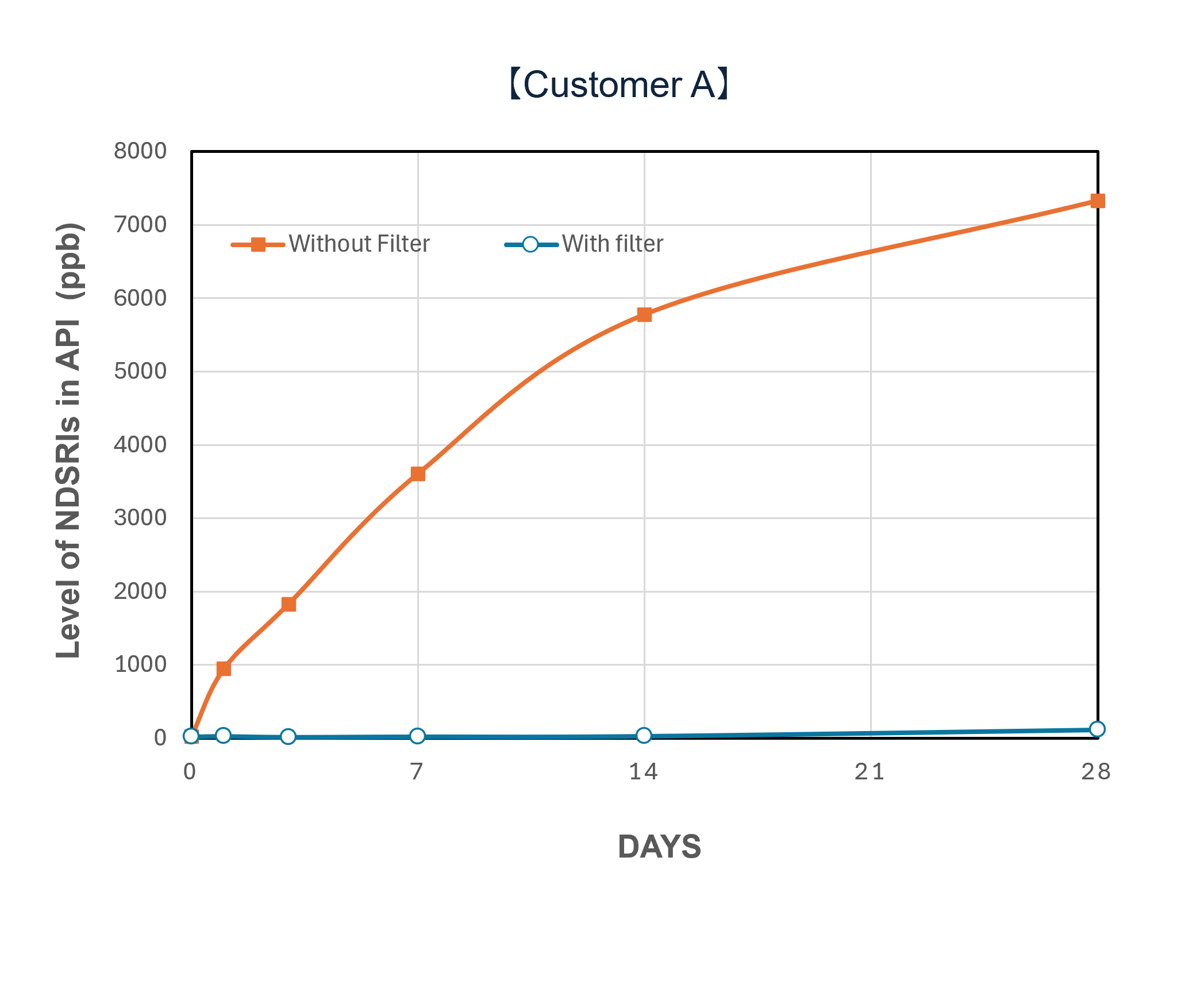
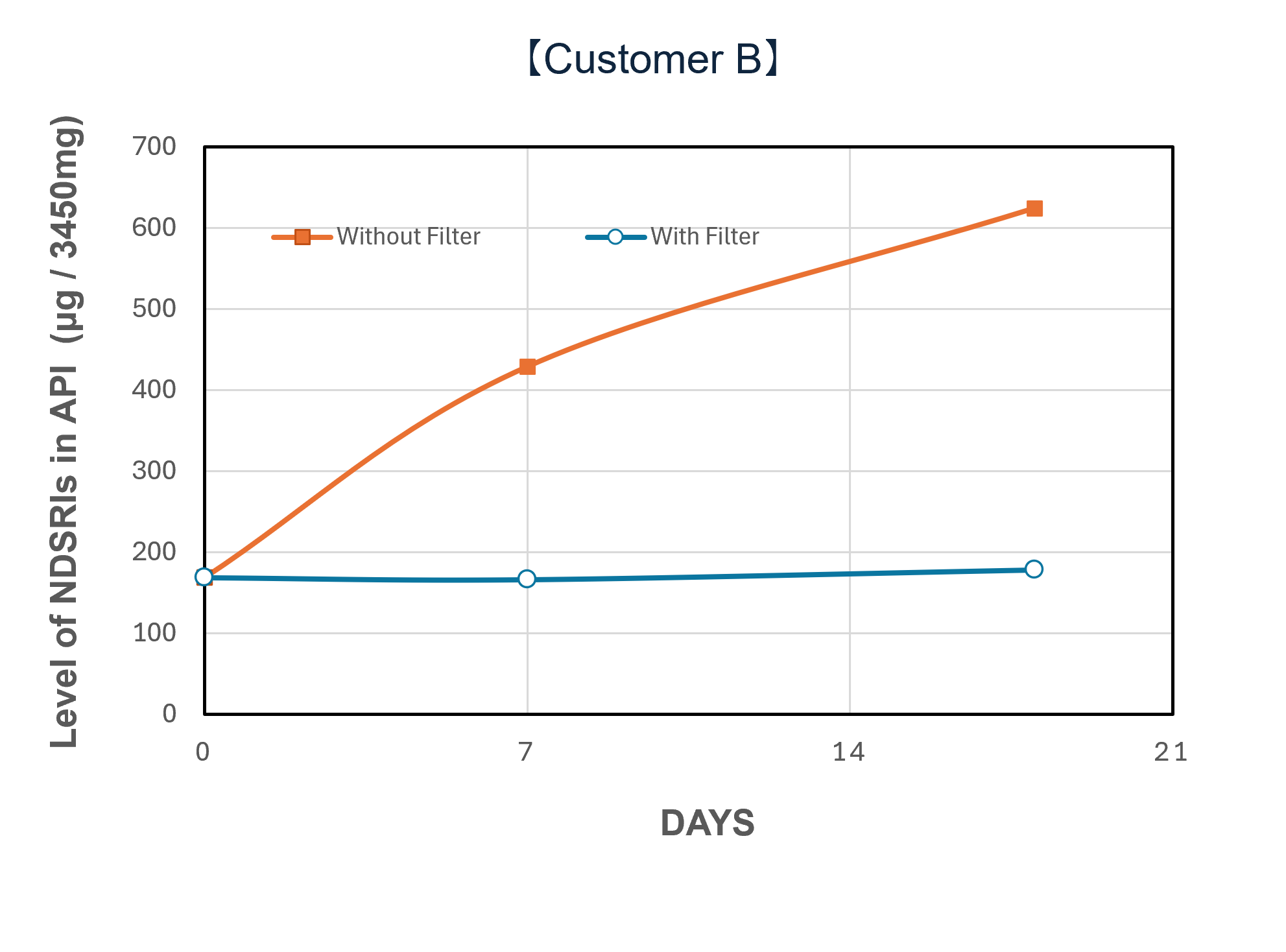
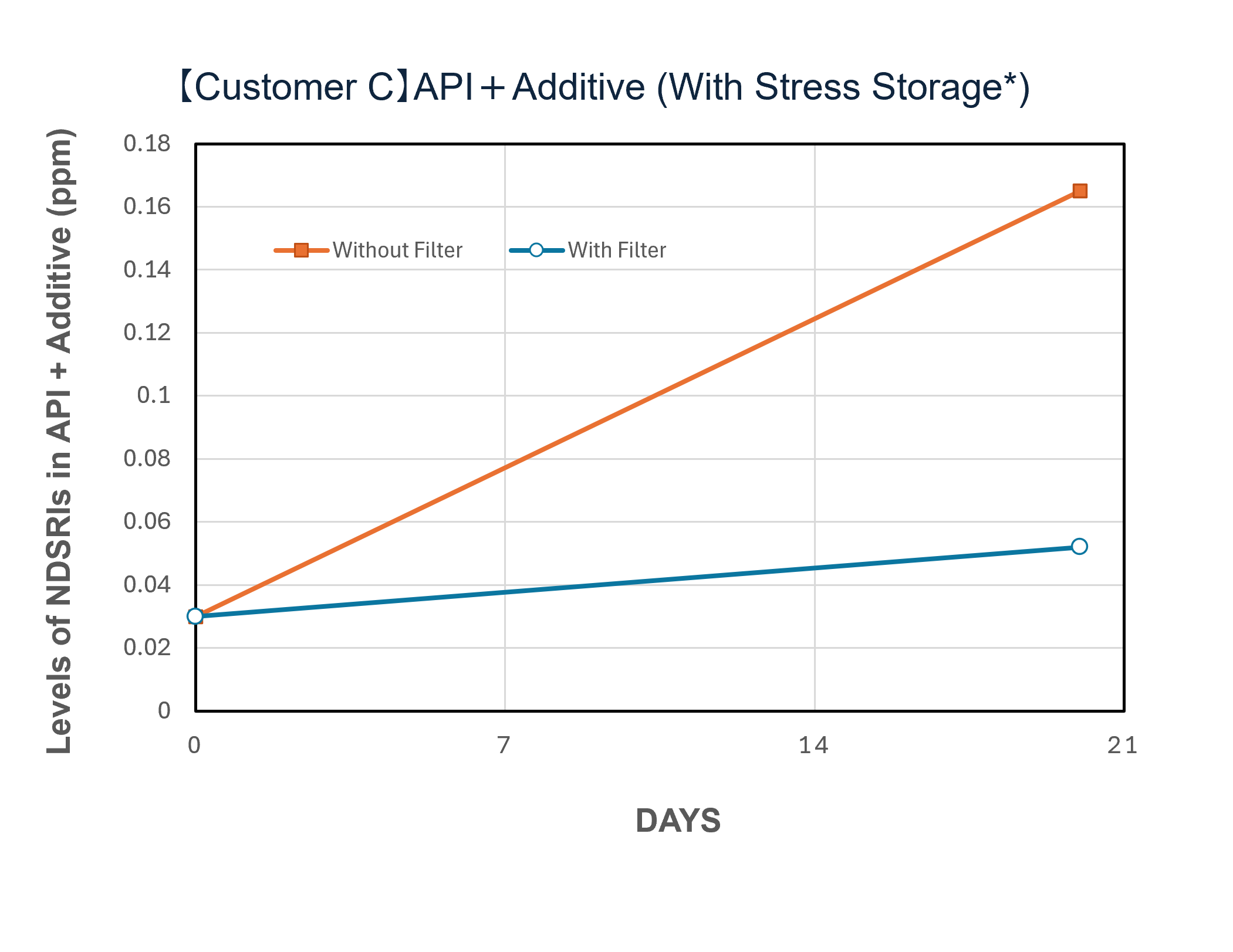
Demonstration Cases and Evaluation Results
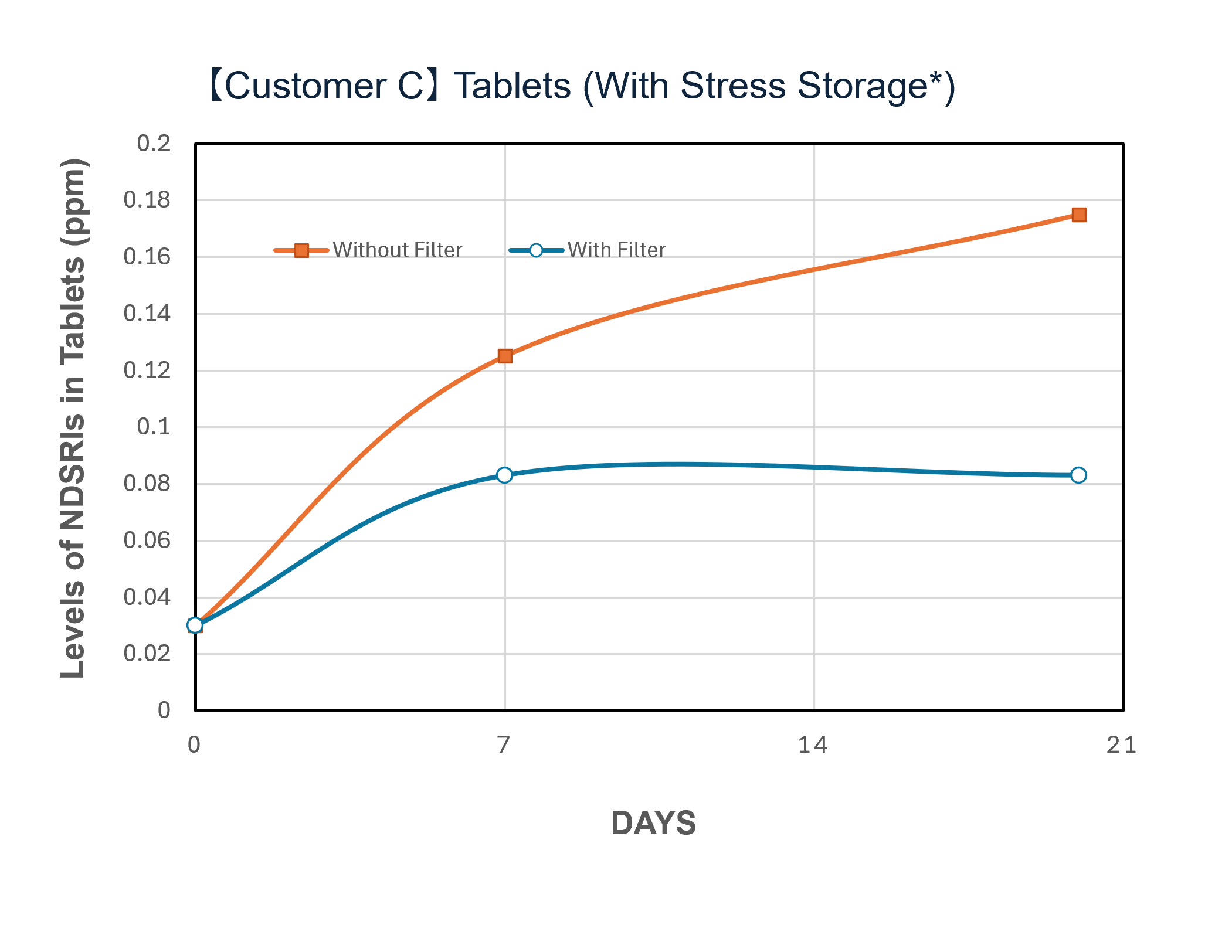
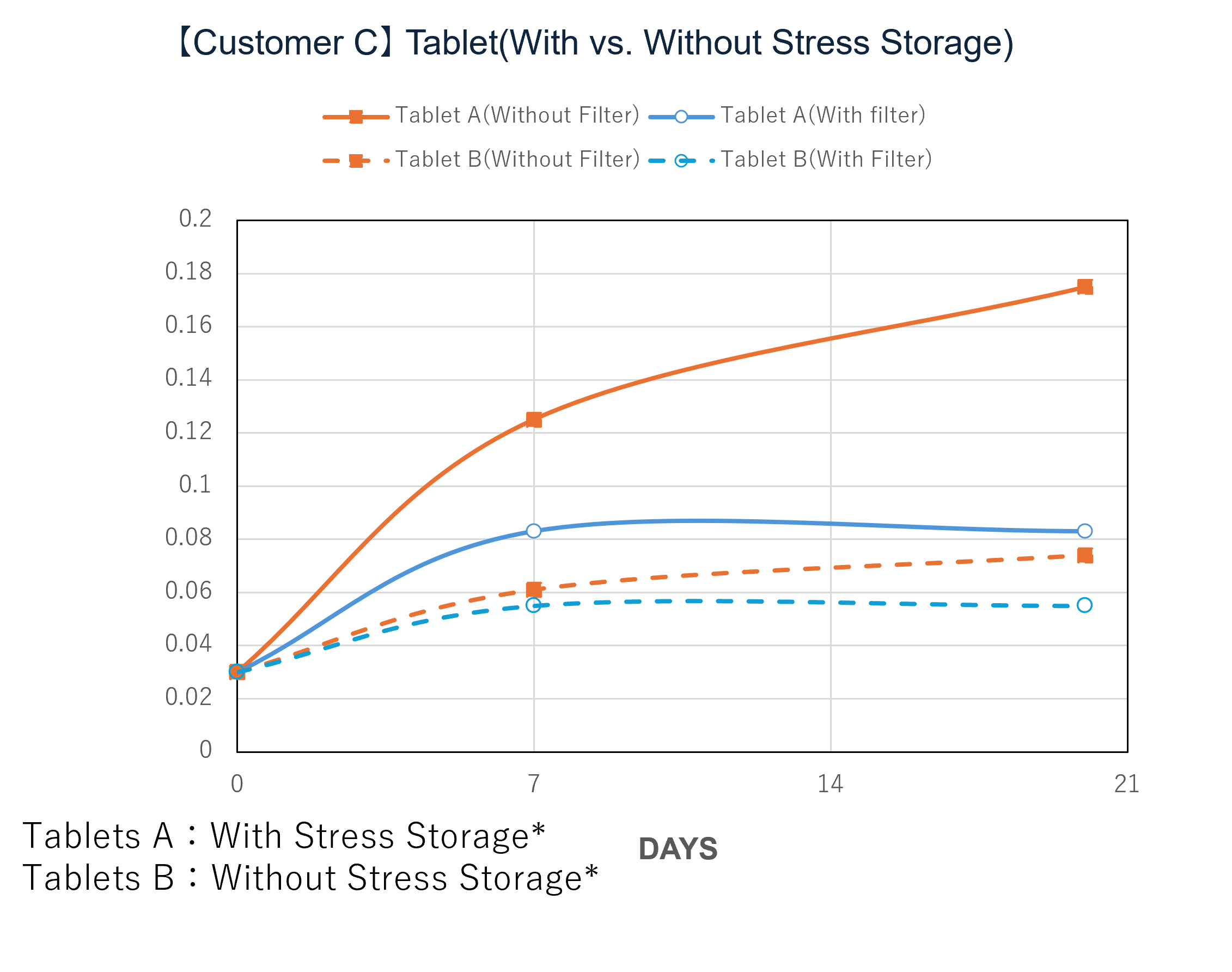
In a demonstration case at a pharmaceutical company in Japan, the introduction of NOx removal gasphase filters significantly reduced ambient NOx levels and successfully lowered the levels of NDSRIs in both APIs and finished products. Furthermore, comparative testing at the pilot scale (test unit) revealed a clear difference in behavior between the 'with filter' and 'without filter' setups, confirming that the filters can mitigate the increase in NDSRIs concentration over time.
| Performance Evaluation of NOx Removal Filter System via Test Unit (Example) | |||
 |
 |
||
 |
|||
 |
 |
||
* Samples were exposed to the air upstream and downstream of the filter for a specified number of days, then stored under sealed conditions at 60℃ for one week.
5. GMP Compliance & Quality Excellence — Risk Management Strategies for Manufacturing Environments
To ensure pharmaceutical quality, it is essential not only to manage raw materials and equipment but also to optimize the manufacturing environment itself in accordance with GMP. Recent quality challenges, such as the nitrosamine issue, highlight the need to focus on factors outside the direct manufacturing process—elements that were previously often overlooked—such as ambient air quality, cross-contamination from shared facilities, and the atmospheric conditions during packaging and storage.
In response to these challenges, there is an accelerating shift toward bringing the surrounding environment under rigorous control.
Consequently, air quality management and NOx removal systems are no longer seen as mere capital investments; they are being repositioned as vital risk management tools for GMP compliance and quality assurance. Comprehensive quality control leveraging environmental technologies will be the key to achieving 'preventative quality assurance' in the future of pharmaceutical manufacturing.
Conclusion
Nitrosamine impurities represent a major quality risk that pharmaceutical companies must navigate as regulations become stricter. The complexity of these risks—arising from synthesis to storage—means that simply changing manufacturing methods or dedicating equipment is no longer enough.
In this context, Nippon Muki’s NOx removal gasphase filter system offers a practical and complementary solution by targeting the manufacturing environment. By taking a holistic view of the production process and controlling 'invisible contamination sources' like ambient air, this represents a novel approach to supporting quality assurance, GMP compliance, and risk mitigation. As regulatory scrutiny intensifies, the necessity for a comprehensive approach that includes advanced environmental control will only continue to grow.
Reference
・FDA ‘Information about nitrosamine impurities in medications’ U.S. Food and Drug Administration+1
・Review ‘Nitrosamine impurities in pharmaceuticals: An empirical review…’ PubMed
・EMA ‘Nitrosamine impurities in human medicines’ European Medicines Agency (EMA)+1


Comment
/
/
/
Comment